Viscoll® Collagen is offered for customized formulation of medical-grade collagen-based biomaterials
Viscoll® Collagen is a new line of multi-functional products made from highly purified type I collagen in a sterile and concentrated solution. The wide range of Viscoll® Collagen concentrations allows the creation of materials with controllable mechanical properties compared to soft tissues. A notablel feature of Viscoll® Collagen is its high adaptability to the requirements of a large number of modern methods used in regenerative medicine and tissue engineering, such as 3D bioprinting.




Applications
Viscoll® Collagen is a versatile material adaptable to meet any application challenges in the field of regenerative medicine and tissue engineering.
Viscoll® Collagen can be used in projects aiming to translate research into clinical practice.
Some proven areas of application are listed below. Please contact us for additional information.
Cell transplantations
Viscoll® «ready-to-use» solutions are offered by IMTEK. Our experts will help develop a new product for your needs.
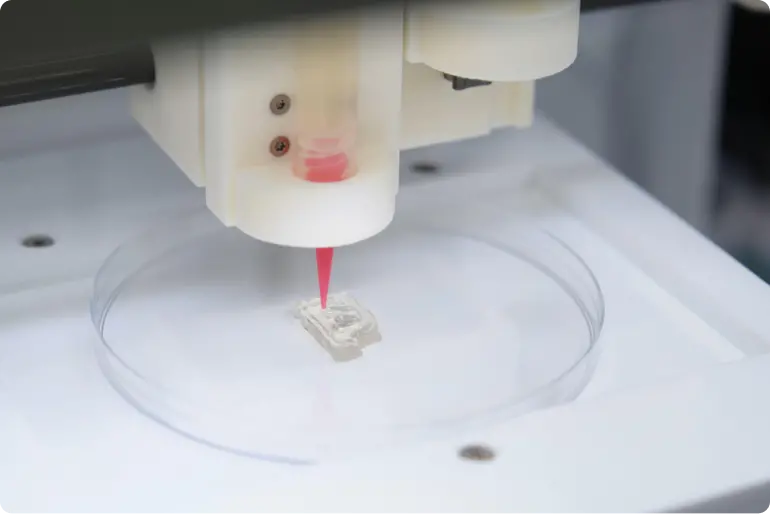
3D Bioprinting
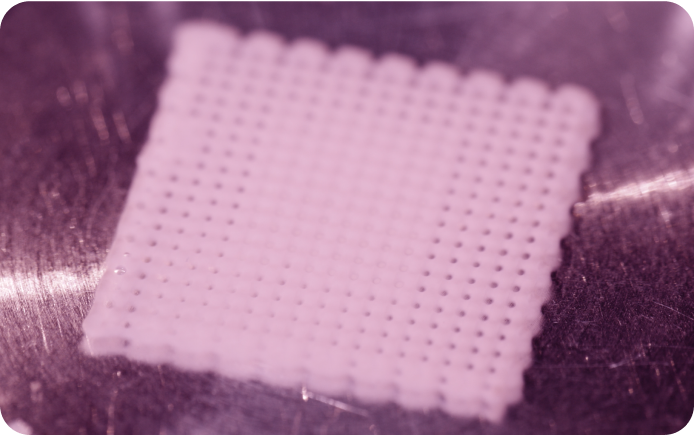
Create «cell-laden» collagen hydrogels with complex geometries and defined pore sizes using direct extrusion 3D bioprinting and Viscoll® Collagen.
Research & Development
IMTEK also offers a series of highly purified extracellular matrix (ECM) proteins (collagen types I-V; fibronectin; vitronectin; laminin). You can make your own ECM by combining these components with Viscoll®.
Key features of Viscoll®Collagen
- High biocompatibility, low immunogenicity and safety
- Ability to form stable and strong structures at neutral pH and temperature of 37°C
- Supporting long-term cell survival during their in vitro culturing
- Possibility of being used as bio-inks for 3D bioprinting
- Adaptability to other methods of structure formation (molding, electrospinning, etc.)
The product format is adapted for quick creation of extracellular matrix necessary for long-term cell culturing.











collaboration
Our team is open to collaboration with scientific teams and clinical centers.
The goal is the development of new implantable collagen biomaterials and their translation to clinical practice.