When developing a matrix for matrix-assisted cell transplantation, researchers inevitably face the problem of choosing a biopolymeric matrix that must ensure the survival of the cells contained in it before and after their introduction into the living organism. Polymeric materials serving as the basis for the formation of such a matrix structure must be compatible with both the cells included in them and in the living tissues. They should slowly decompose in the body without turning into harmful components and gradually being replaced by an extracellular matrix synthesized by the organism's own cells. Such polymeric materials, as well as the products of their decomposition, should not be immunogenic.
What is Viscoll® collagen?
Among the large number of natural and synthetic polymers, native highly purified collagen is biocompatible and does not cause a virus-specific immune response, as is the characteristic for other proteins and biopolymers. Therefore, collagen biomaterials are actively used in clinical practice. However, the collagen preparations available on the market for the manufacture of biomaterials have a significant limitation: they form mechanically weak structures that are difficult to manipulate.


- High biocompatibility, low immunogenicity, and safety
- Ability to form stable and strong structures at a neutral pH and a temperature of 37°C
- Supporting long-term cell survival during their in vitro culturing
- Possibility of being used as bio-inks for 3D bioprinting
- Adaptability to other methods of structure formation (molding, electrospinning, etc.)
Viscoll® collagen is produced in accordance with the requirements of ISO 13485 "Medical devices. Quality management systems. Requirements for regulatory purposes" as well as the requirements of ISO 22442 "Medical devices using animal tissues and their derivatives". Therefore, Viscoll® collagen can be used both for the manufacture of medical devices and for the matrix-assisted cell transplantation.
Advantages of concentrated VISCOLL® collagen solutions
Production of Viscoll® Collagen
Viscoll® Collagen is produced at the production site of "Imtek" Ltd. (Russia). The production is certified according to ISO 13485, "Medical Devices. Quality Management Systems. Requirements for Regulatory Purposes", and also complies with the requirements of ISO 22442 "Medical Devices Using Tissues and Their Derivatives of Animal Origin". The production is localized in the Moscow region at the laboratory-production complex of "Imtek" Ltd. with an area of 1100 sq.m., equipped with supply and exhaust ventilation, provided with 23 automated ventilation systems with control of the aerodynamic mode of the premises, and a three-stage air purification system. Control measures have been introduced at the production site to separate operations and to minimize the risk of possible contamination. There are rooms for raw material preparation, production in clean rooms of ISO-7 and ISO-5 classes, quality control, cell culturing, and a research laboratory.


Medical devices based on VISCOL®Collagen
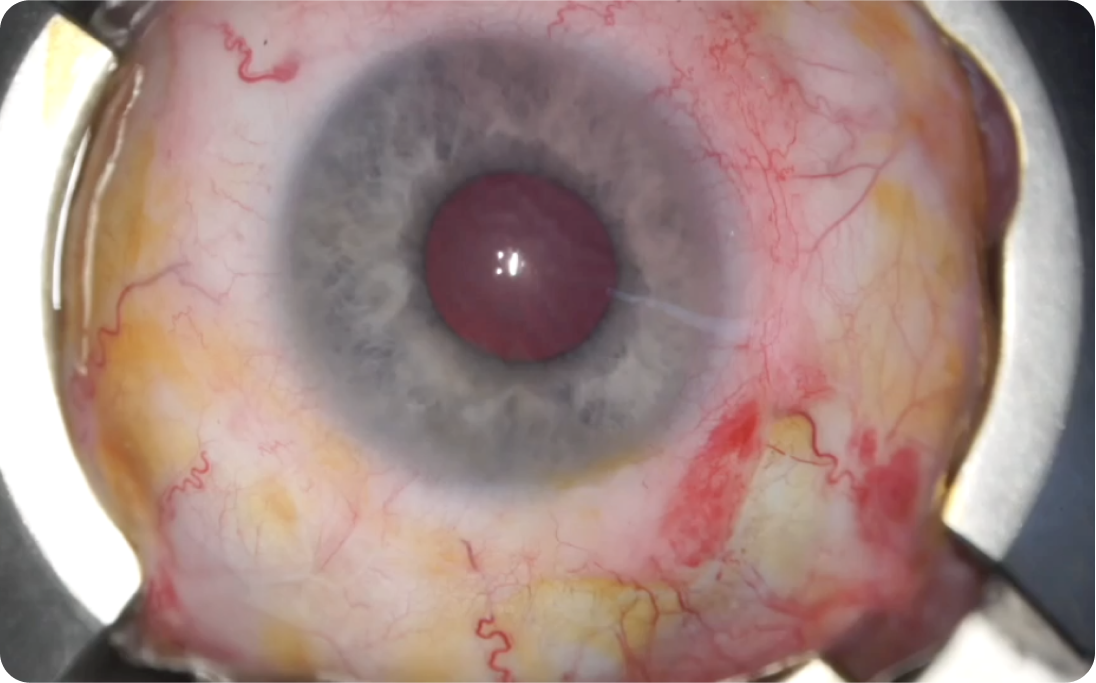
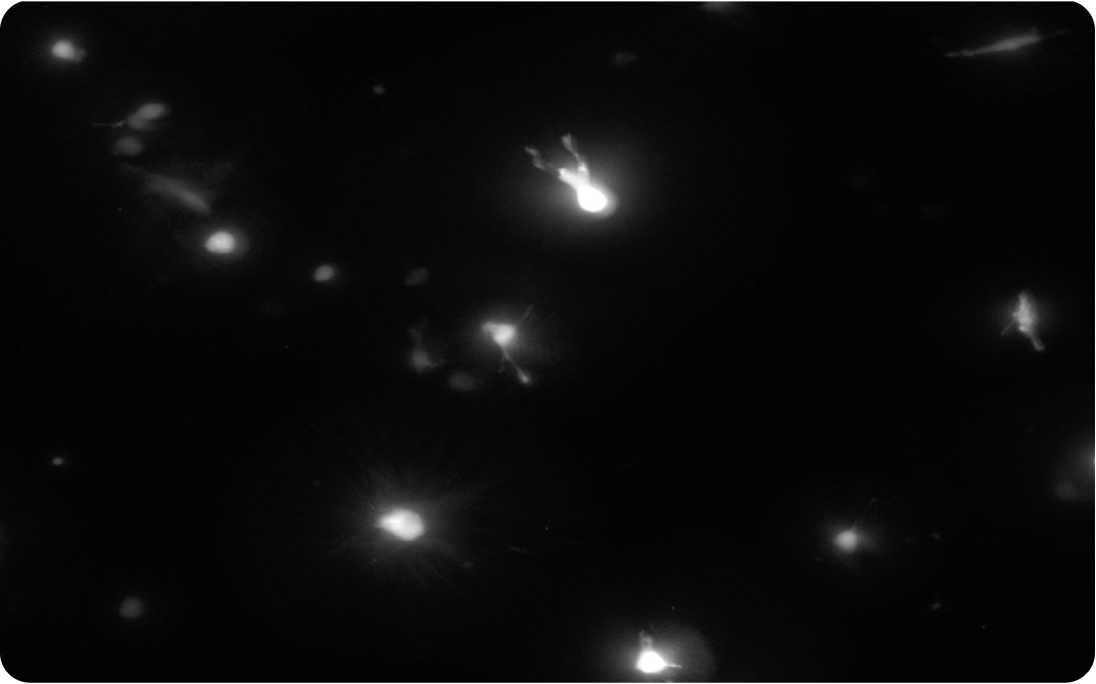
Perspective for cell transplantation