Medical product description
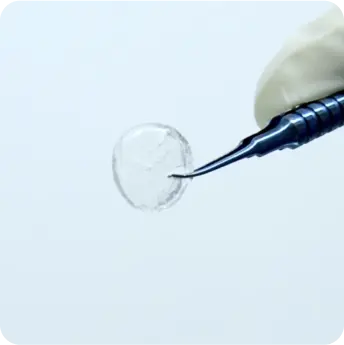
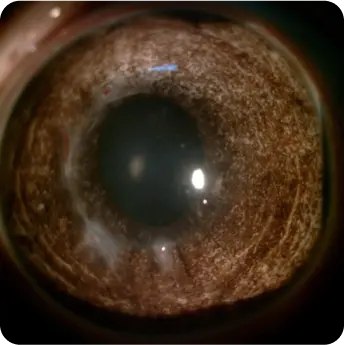
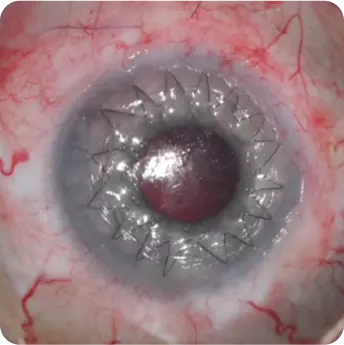
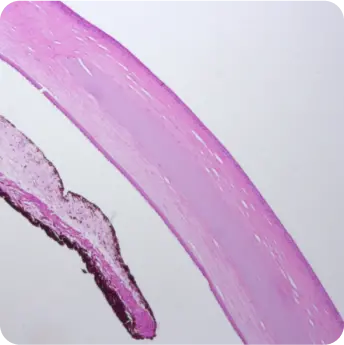
The product is a homogeneous, rounded, transparent collagen plate made from highly purified native type I collagen isolated from porcine tendons. The product is intended to replace the surface of the eye through surgical intervention. The product can be used for anterior layer-by-layer and reinforcing keratoplasty for treatment of corneal leukoma and ulcers of various etiologies, pterygium, as well as for surgical treatment of traumatic injuries of the eyeball. And also to use as a temporary keratoprosthesis for surgical manipulations in the absence of corneal transparency.